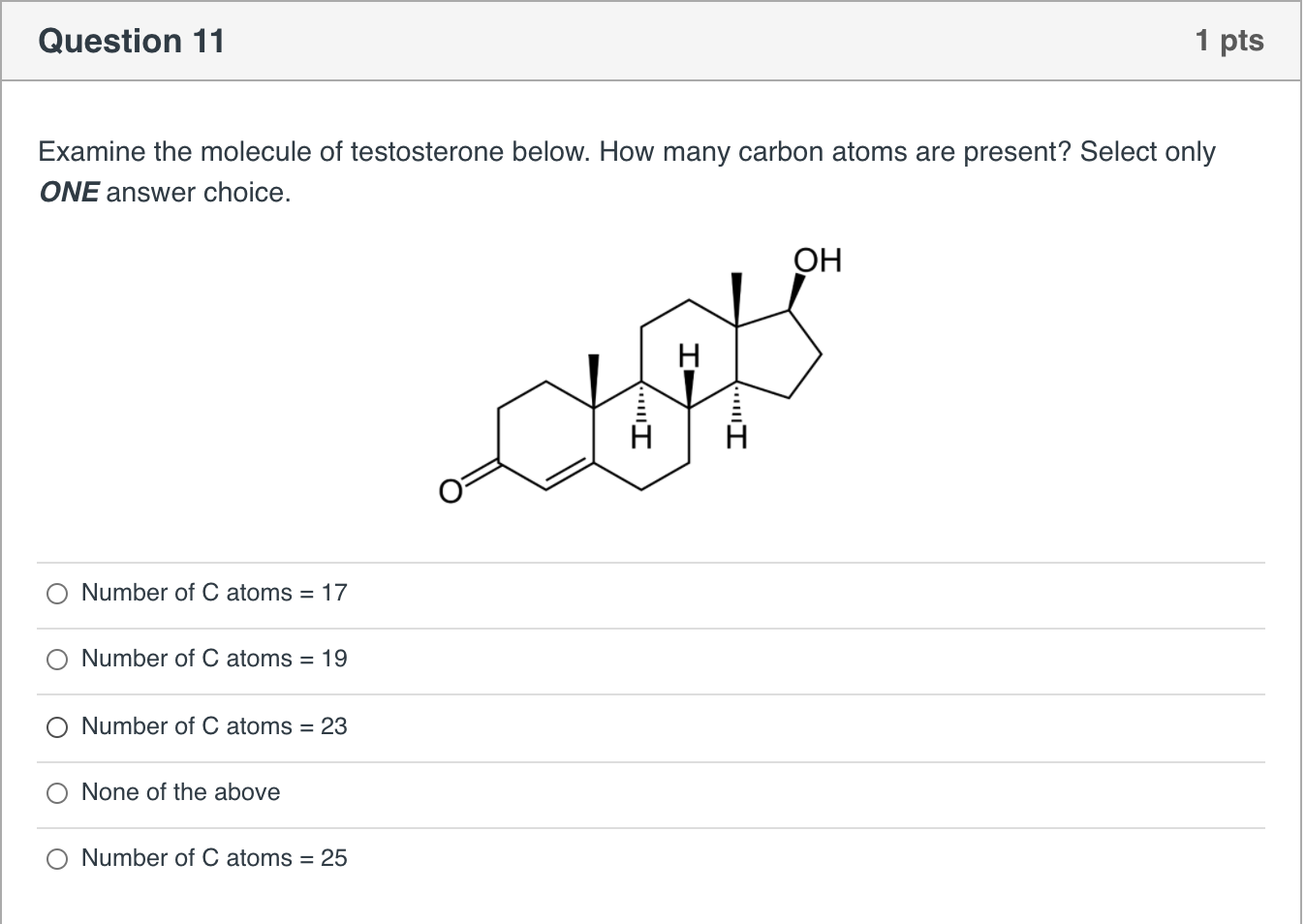
How Many Atoms of Carbon Are in C19h28o2
1200 g C-12 1 mol C-12 atoms 6022 x 10 23 atoms. B How many molecules of testosterone does it contain.

How To Convert Atoms To Molecules Moles And Grams Youtube
126 1024 carbon atomsD.

. A sample of male testosterone C19H28O2 contains 3881021 hydrogen atoms. Element Carbon C Group 14 Atomic Number 6 p-block Mass 12011. A sample of the male sex hormone testosterone C19H28O2 contains 388 1021 yhydrogen atoms.
How many moles of testosterone does it contain. A sample of the male sex hormone testosterone C19H28O2 contains 3781021 atoms of hydrogen1How many atoms of carbon does it contain2How many molecules of testosterone does it contain3How many moles of testosterone does it contain4What is the mass of this sample in grams. The chemical symbol for Carbon is C.
Number of hydrogen atoms in C19H28O2 388 X 1021 atoms. 126 1023 carbon atomsC. To find atoms of carbon we take the number of hydrogen atoms and multiply the number by 1928 ratio of carbon to hydrogen atoms thus cancelling the number of hydrogen atoms and getting carbon atoms.
This molecule turned out to be a soccer-ball-shaped sphere made of 60 carbon atoms. Solution for Acetaldehyde has the molecular formula C2H4O. In this question we have to find out 4 answers A sample of male sex hormone testosterone C19H28O2 contain 348 x 1021 atoms of Hydrogen.
A To find out the atoms of carbon does it contains we take the number of hydrogen. How many Atoms do Carbon have. Atomic carbon systematically named carbon and λ 0-methane also called monocarbon is a colourless gaseous inorganic chemical with the chemical formula C also written C.
It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. Vitamin A plays an essential role in many physiologic processes including proper functioning of the. A How many atoms of carbon does it contain.
The research team named their discovery the buckminsterfullerene after an architect who designed geodesic domes. A sample of male testosterone C19H28O2 contains 3881021 hydrogen atoms. How many molecules of testosterone does it contain.
Atomic carbon is the simplest form of carbon and is also the progenitor of. How many atoms of carbon. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms.
Express your answer in atoms to three significant figures. Sources facts uses scarcity SRI podcasts alchemical symbols videos and images. It is kinetically unstable at ambient temperature and pressure being removed through autopolymerisation.
Solution for A sample of the male sex hormone testosterone C19H28O2contains 388 1021 hydrogen atoms. Retinol is the fat soluble vitamin retinol. To find the number of molecules we use the ration of 128 where 1 molecules of C19H28O2 has 28 H atoms.
How the structure is described will depend on the process used. A How many atoms of carbon does it contain. Find the number of moles of hydrogen in.
6023 1024 carbon atoms. Carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure. D What is the mass of this sample in grams.
The nucleus is composed of protons and neutrons. A sample of the male sex hormone testosterone C19H28O2 contains 388 1021 hydrogen atoms. How many carbon atoms are present in 035 mol of C 6 H 12 O 6A.
Carbon makes up only about 0025 percent of Earths crust. A sample of the male sex hormone testosterone C_19H_28O_2 contains 388 times 10 21 hydrogen atoms. Vitamin A binds to and activates retinoid receptors RARs thereby inducing cell differentiation and apoptosis of some cancer cell types and inhibiting carcinogenesis.
Atomic number6 atomic weight120096 to 120116 melting point3550 C 6420 F boiling point4827 C 8721 F density diamond352 gcm3. View the full answer. Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a.
A sample of the male sex hormone testosterone C19H28O2 contains 388 1021 yhydrogen atoms. Carbo coal is a chemical element with the symbol C and atomic number 6. C How many moles of testosterone does it contain.
Avogadros Number of Particles is called the number of particles in 1 mole 60221421 x 10 23. Carbon-14 which is radioactive is the isotope used in radiocarbon dating and radiolabeling. Some of the most common carbon compounds are.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. How many atoms of carbon. A How many atoms of carbon does it contain.
How many atoms of carbon does it contain. A sample of the male sex hormone testosterone C19H28O2 contains 39810 21 atoms of hydrogen. Many carbon compounds are essential for life as we know it.
How many atoms of carbon does it contain. A How manyatoms of carbon does it contain. Jump to main content.
Carbon dioxide CO 2 carbon monoxide CO carbon disulfide CS 2 chloroform CHCl 3 carbon tetrachloride CCl 4 methane CH 4 ethylene C 2 H 4 acetylene C 2 H 2 benzene C 6 H 6 ethyl alcohol C 2 H 5 OH and acetic acid. 6023 1023 carbon atomsB. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Given 550 g of Acetaldehyde answer the following questions.

Solved A Sample Of Testosterone C19h28o2 Contains 7 08 10 20 Hydrogen Atoms I How Many Carbon Does It Contain Ii How Many Molecules Does It Contain Iii How Many Moles Of Testosterone Does It Contain

A Sample Of The Male Sex Hormone Testosterone C19h28o2 Contains 3 88 10 21 Hydrogen Atoms
Comments
Post a Comment